Disease-modifying Medicines: Hope or Hype? Part 3
I was recently asked to take part in a panel discussion hosted by the European Parkinson Therapy Centre. We were asked to provide rationale for the potential of different “medicines” to change or modify disease progression. The “medicines” discussed included pharmaceuticals, gene therapies, nutrition and exercise. There were several different questions for the panelists. I have summarized my answers in defense of exercise as medicine for one questions below with more to come in future newsletters! You can also read Question 1 and 2 linked below.
This is GREAT information that you can use to educate and empower your clients and community about what IS possible with exercise. Become an advocate for change! Raise awareness of the need for PD-specialized rehabilitation and community exercise professionals working collaboratively to increase access to “exercise as medicine” for LIFE! That is OUR MISSION at PWR and it was so much fun to participate in this webinar to raise world-wide awareness of WHAT we already do and WHY.
Panelists included:
Simon Stott (Moderator) from Cure Parkinson’s; Thomas Foltynie from University Central London; James Beck from Parkinson Foundation; Laurie Mischley from Bastyr University; Becky Farley from Parkinson Wellness Recovery (PWR)

To read part 1 and 2
Question #3 If exercise is medicine for PwP........... Is it Preventative? Disease-modifying? Symptomatic?
That depends..... upon when you start to exercise, the dose (volume and intensity) and, how long you sustain practice, your risk factors like genetics, environmental exposures to contaminants, lifestyle......AND the ability to measure disease onset and severity!
Let’s start with the prevention of PD.
What does that mean? Is it possible with exercise or other interventions?
Physical exercise is already officially recognized “as medicine” for numerous chronic diseases based upon its’ effect on pathogenesis and symptoms. Exercise-induced positive brain change (neuroplasticity) modulates many of the same cellular mechanisms that are involved in the pathophysiology of aging and other neurodegenerative diseases like Alzheimer’s and Parkinson disease. For example, exercise improves autophagy, reduces oxidative stress, promotes mitochondrial biogenesis and neurotrophic factors (Figure 1) as well as stimulates angiogenesis, metabolism and reduces inflammation. Exercise also induces structural changes in the brain in animals and humans that benefit basal ganglia circuits and behavior through increased dopamine release, improved connectivity (excitability), restored dopamine terminals, increased dopamine D2 receptor binding and weakening of overactive pathways interfering with normal dopamine signaling.
But can exercise-induced neuroplasticity also induce changes that are preventative for a neurodegenerative disease like PD? If so, it would theoretically be expected to reduce the incidence and prevalence of PD! Sounds crazy right? The answers depend upon one’s lifetime history of exercise prior to diagnosis of PD, as well as one’s genetics, environmental exposures and other lifestyle factors (i.e., sleep, stress, diet, socialization). In humans, studies recruit large numbers of healthy populations in their midlife (~ 50’s); track each person’s volume and intensity of exercise and follow them for 10, 20 or more years to calculate how many people convert to a diagnosis of PD!
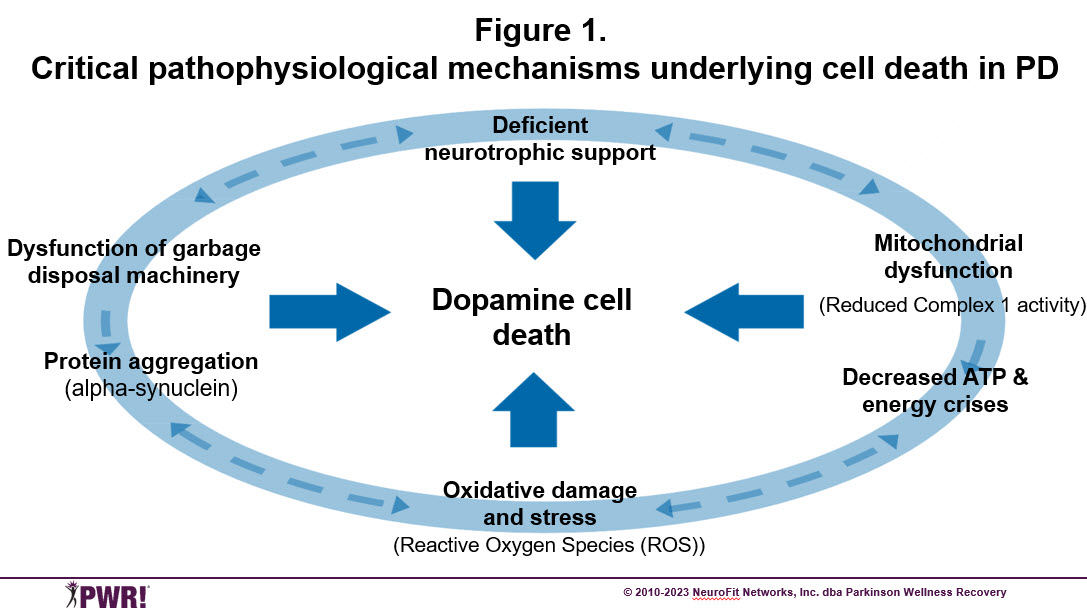
While these type of prevention studies do exist, they don’t provide much insight on “mechanisms.” In the future they may be able to include individuals with genetic risks or environmental exposures to see if that influences risk or rate of progression. As new biomarkers become available, they may be able to identify PD earlier in the prodromal phase, so they don’t have to wait so long and can help identify individuals that will be good candidates for future preventative or disease-modifying studies (See Figure 2). Unlike humans, animals don’t get PD naturally, so researchers use toxins to kill dopamine neurons that are quick-acting (~ 24 hours) such that exercise usually occurs 7-14 days before and after toxin exposure (as compared to 10-30 years in human studies). Recently, animals with genetic mutations related to triggering known PD pathology, have been studied, which have a more gradual onset of disease. While animal studies have limitations, the advantages are that it is much easier to manipulate dosage, type of exercise, severity of disease and to directly quantify effects of exercise on disease pathology. And, you don’t have to wait 10-30 years to know the results!
Fig. 2 Illustration of the progression of Parkinson disease and how any potential disease-modifying interventions are time-dependent
Pre-diagnostic Stages
- Preclinical phase – Asymptomatic, high-risk individuals; aberrant pathophysiological mechanisms may be in play, but dopamine system not yet compromised
- Prodromal phase - Dopamine cell death begins, only nonmotor symptoms; precedes DX by ~ 10-15 years Stages of Clinically Manifest PD
Early – Moderate – Advanced
- At first motor symptoms, 40-50% of dopamine neurons are lost; by the time of diagnosis, it is more like 50-60%
- Once diagnosed, dopamine therapies are initiated
- Mechanisms of exercise-induced (neuroplasticity) that may underlie prevention, disease-modification or symptomatic benefits
- Neuroprotection: To halt or slow cell death
- Neurorestorative: To improve function of damaged or vulnerable DA neurons and circuits
- Compensation: To shift functions to “spared” neurons and circuits
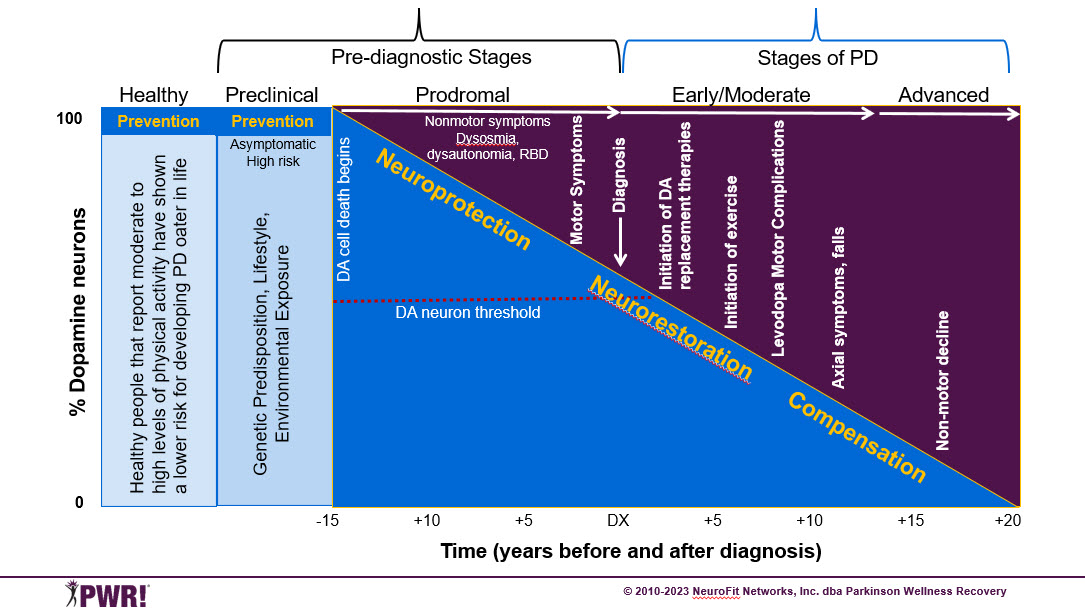
What is the evidence for prevention in animals and humans with PD?
Direct evidence exists in animal models of PD that aerobic and skilled exercise protects the dopamine neurons when implemented immediately before or after exposure to DA toxins in healthy animals or increases autophagy in the preclinical period in high-risk animals with genetic mutations for alpha-synclein (See Figure 2). This suggests that exercise may be able to prevent environmental toxins from killing healthy dopamine neurons or reverse disease pathology that may be triggered by genetic predisposition.
Indirect evidence exists in health individuals from several large observational cohort studies that show higher levels of physical activity (either volume or intensity) is associated with a 30% lower risk of developing PD and decreased all-cause mortality in persons living with PD. People that were active before and after PD diagnosis have the lowest mortality risk. A recent study characterized the baseline difference in basal ganglia connectivity and dopamine release, motor and non-motor symptoms, and function in habitual exercisers vs. sedentary individuals at diagnosed. While it only represents a snapshot in time, the habitual exercisers at diagnosis had a more robust DA system, reduced symptoms and better function compared to their sedentary peers at diagnosis. This suggests they because they had a more robust dopamine system, they may have put off the onset of motor symptoms longer. It also implies that they are the ones most likely to participate in exercise and require less medication after diagnosis. While far from definitive, recommending individuals at high risk for developing PD to engage in exercise to potentially “prime” their dopamine system and put of the motor symptoms longer sounds practical and intuitive.
What about the environment, biomarkers, “sniffer dogs” and prevention?
Parkinson’s is now the fastest growing neurological disorder in the world. Aging populations are growing and environmental contamination is continuing, both known risk factors for developing PD. However, even if you correct for aging, the incidence (frequency of diagnoses) of PD is still more common than Alzheimer’s. Some people refer to this increased incidence as a Parkinson Pandemic. Unfortunately, you can’t blame the higher frequency on new more sensitive diagnostic tests! The diagnosis of PD continues to depend upon observation of signs and symptoms by expert neurologists, just like when Dr. James Parkinson characterized the disease in 1817 during the industrial revolution. So, what is to blame for this pandemic? As an expert in neurotoxicology and the role of environmental contaminants in PD, Dr. Briana De Miranda argues that increased exposure to environmental contaminants is to blame for the increased rate of diagnoses (I am pretty sure Dr. James Parkinson would agree):
In the case of Parkinson’s disease (PD), the environment takes a more central role; pesticides, industrial byproducts, metals, infections, drug use, head trauma, microbiome, and diet are all implicated in PD pathogenesis. In fact, as approximately 85% of individuals who develop PD are considered idiopathic, Parkinson’s is likely the most environmentally influenced neurodegenerative disease in adults.” World Parkinson Congress Blog posted March 29th, 2021
To learn more about the role of the environment in PD, read the book “Ending Parkinson’s Disease” by a group of renowned MDS neurologists, listen to webinars by those same authors interviewing environmental scientists and PwP with known exposures to contaminants, or read Dr. Ray Dorsey’s daily “listening tour”, documenting his findings as he traveled from one contaminated community to another across the US (several in Arizona) -- you too may become an environmental activist (PDAvengers.org)!
In response to the call to action put forward in the book, there is now a new January 2022 scientific publication in the Journal of Parkinson’s Disease called “Preventing Parkinson’s Disease: An Environmental Agenda.” You can click on these links to read the publication, watch a short webinar or read a World Parkinson Congress (WPC) blog summarizing the rationale and priorities for an environmental research agenda. Most recently, on March 30th, 2023, a Bipartisan National Plan to End Parkinson’s Bill was reintroduced. This is the first-ever legislation solely devoted to ending Parkinson’s disease. Your care read about it here.
While removing environmental contaminants is an important goal towards prevention, there are other new and emerging therapies that may have a role in prevention, including gene or cell therapies, new or repurposed drugs. However, for these “prevention” studies to be successful, there must be a way to identify individuals at risk for PD through genetic screening and documentation of environmental exposure before disease pathology begins (See Figure 2). That will allow new therapies (including exercise) to start earlier, in the pre-diagnostic stage, before or right after dopamine cell death begins when it may be possible to put off the onset of PD (prevention) and/or slow its’ progression immediately after it starts (disease modification). Fortunately, MJF just released breaking news (4.13.2023) about a new “biomarker” tool that can detect abnormal alpha synuclein in “at risk” individuals with varying genetic predisposition or in prodromal nonmotor risk factors (i.e., REM sleep disorder, loss of smell). Not only is that biomarker important for identifying high risk individuals for prevention studies of PD, but it may also be useful for measuring disease progression. WOW!
Finally, what about dogs? Can they play a role in early detection; like a living biomarker detector? Instead of taking a sample of your spinal cord fluid, you may be asked to provide an article of clothing and allow special dogs to “sniff out PD!” Has Parkinson’s gone to the dogs? Check it out for yourself. Here are some great resources from a WPC blog by Dr. Laurie Mischley in 2018, about her nonprofit “Pads for Parkinson’s” in Washington and a news story about their mission to train dogs to sniff out PD.
Stay tuned for the next release when we will consider
If exercise is medicine for PwP...........
Is it disease-modifying?

References
- Chen H, Zhang SM, Schwarzschild MA, Hernán MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64(4):664-669. doi:10.1212/01.WNL.0000151960.28687.93
- Crotty GF, Keavney JL, Alcalay RN, et al. Planning for Prevention of Parkinson Disease. Neurology. 2022;99(7 Supplement 1):1-9. doi:10.1212/WNL.0000000000200789
- Crotty GF, Schwarzschild MA. Chasing Protection in Parkinson’s Disease: Does Exercise Reduce Risk and Progression? Front Aging Neurosci. 2020;12:186. doi:10.3389/fnagi.2020.00186
- Ellis T, Rochester L. Mobilizing Parkinson’s Disease: The Future of Exercise. Brundin P, Langston JW, Bloem BR, eds. J Parkinsons Dis. 2018;8(s1):S95-S100. doi:10.3233/JPD-181489
- Garcia Ruiz PJ, Luquin Piudo R, Martinez Castrillo JC. On Disease Modifying and Neuroprotective Treatments for Parkinson’s Disease: Physical Exercise. Front Neurol. 2022;13:938686. doi:10.3389/fneur.2022.938686
- Janssen Daalen JM, Schootemeijer S, Richard E, Darweesh SKL, Bloem BR. Lifestyle Interventions for the Prevention of Parkinson Disease. Neurology. 2022;99(7 Supplement 1):42-51. doi:10.1212/WNL.0000000000200787
- Johnson ME, Stecher B, Labrie V, Brundin L, Brundin P. Triggers, Facilitators, and Aggravators: Redefining Parkinson’s Disease Pathogenesis. Trends Neurosci. 2019;42(1):4-13. doi:10.1016/j.tins.2018.09.007
- Paul KC, Chuang YH, Shih IF, et al. The association between lifestyle factors and Parkinson's disease progression and mortality. Mov Disord. 2019;34(1):58-66. doi:10.1002/mds.27577
- Pedersen BK, Saltin B. Exercise as medicine - Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sport. 2015;25:1-72. doi:10.1111/sms.12581
- Sacheli MA, Murray DK, Vafai N, et al. Habitual exercisers versus sedentary subjects with Parkinson’s Disease: Multimodal PET and fMRI study. Mov Disord. 2018;33(12):1945-1950. doi:10.1002/mds.27498
- Tsukita K, Sakamaki-Tsukita H, Takahashi R. Long-term Effect of Regular Physical Activity and Exercise Habits in Patients With Early Parkinson Disease. Neurology. 2022;98(8):e859-e871. doi:10.1212/WNL.0000000000013218
- Xu Q, Park Y, Huang X, et al. Physical activities and future risk of Parkinson disease. Neurology. 2010;75(4):341-348. doi:10.1212/WNL.0b013e3181ea1597
- Zhang X, Molsberry SA, Schwarzschild MA, Ascherio A, Gao X. Association of Diet and Physical Activity With All-Cause Mortality Among Adults With Parkinson Disease. JAMA Netw Open. 2022;5(8):e2227738. doi:10.1001/jamanetworkopen.2022.27738
Link to Panelist Discussion
European Parkinson Therapy Centre - Disease modifying therapies… hope or hype?
Moderator - Prof Simon Stott, Cure Parkinson's, Cambridge University 208 years and still no drugs/therapy that can stop our condition. What's the problem?
GUESTS- Jim Beck (Parkinson’s Foundation), Tom Foltynie (UCL), Laurie Mischley (Bastyr), Becky Farley (PWR!)
Dr. Becky Farley About the Author
If you ask her, Dr. Becky Farley will tell you that working with her first client with Parkinson’s almost 20 years ago changed her life and her career, and since then she hasn't stopped changing lives of those living with Parkinson's, in Arizona and the rest of the world. In 2010, she established the PWR!Gym in Tucson and has been offering people with Parkinson disease the PD-specific physical therapy, group exercise, and social engagement they need to get better and stay better. Globally, Dr. Farley supports PWR!’s mission of making cutting-edge PD-specific "exercise as medicine” available to the 10 million people living with PD worldwide, by training healthcare and fitness professionals in the PWR!Moves and the PWR!4Life model (and she’s trained over 6000 professionals since she started in 2010!).
When she’s home in Arizona, you’ll find Becky working on Parkinson Disease-specific exercise at the PWR!Gym as well as leading our annual exercise intensive PWR! Retreats. When she’s on the road, she’s either teaching our PWR!Moves workshops or giving talks at conferences, community organizations, and healthcare networks across the US and world.
When she isn’t working, you can find her working out, walking the dog, and gardening. To learn even more about Dr. Farley, visit our team page.

